Varicella-Zoster Virus
Chickenpox (Varicella)
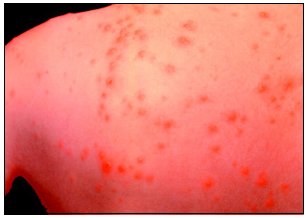
- Chickenpox is caused by varicella-zoster virus (VZV) and is usually mild, but it may be severe in infants, adults, and persons with impaired immune systems.
- Almost everyone gets chickenpox by adulthood (more than 95% of Americans). Chickenpox is highly contagious. Approximately 4 million cases occur in the United States each year.
- The virus spreads from person to person by direct contact, or through the air. Approximately 90% of persons in a household who have not had chickenpox will get it if exposed to an infected family member.
- The greatest number of cases of chickenpox occurs in the late winter and spring.
- Chickenpox has a characteristic itchy rash, which then forms blisters that dry and become scabs in 4-5 days. The rash may be the first sign of illness, sometimes coupled with fever and general malaise, which is usually more severe in adults. An infected person may have anywhere from only a few lesions to more than 500 lesions on his or her body during an attack (average 300-400).
- Adults are more likely to have a more serious case of chickenpox with a higher rate of complications and death.
- Chickenpox is contagious 1-2 days before the rash appears and until all blisters have formed scabs. Chickenpox develops within 10-21 days after contact with an infected person.
- Every year there are approximately 5,000-9,000 hospitalizations and 100 deaths from chickenpox in the United States.
- Varicella vaccine has been available since March 1995 and is approved for use in healthy children 12 months of age or older, and susceptible (i.e., no evidence of having had chickenpox in the past) adolescents and adults.
- Varicella vaccine is highly effective in protecting against severe chickenpox. Cases of disease caused by the wild virus, which may occur in a small proportion of vaccinees, are typically very mild, with fewer than 50 skin lesions and no fever.
- It is recommended that all children be routinely vaccinated at 12-18 months of age and that all susceptible children receive the vaccine before their 13th birthday (CDC Advisory Committee on Immunization Practices, the American Academy of Pediatrics and the American Academy of Family Physicians). Many states will require vaccination for entry into pre-school or public school beginning in 1999. The vaccine is also approved for susceptible adolescents and adults, especially those with close contact with persons at high risk for serious complications (e.g., health-care workers, family contacts of immunocompromised persons).
- A history of chickenpox is considered adequate evidence of immunity.
- A blood test is available to test immunity in persons who are uncertain of their history or who have not had chickenpox. Many of these persons will find that they are immune when tested and thus will not need to be vaccinated.
- Effective medications (e.g., acyclovir) are available to treat chickenpox in healthy and immunocompromised persons (e.g, those with cancer, human immunodeficiency virus/AIDS; those receiving medications that depress the immune system).
- Varicella zoster immune globulin (VZIG), an immune globulin made from plasma of healthy volunteer blood donors with high levels of antibody to VZV, is recommended after exposure for persons at high risk for complications (e.g., immunocompromised persons, pregnant women, premature infants <28 weeks gestation or <1000 grams at birth and premature infants whose mothers are not immune).
- For more information about varicella and other vaccine-preventable diseases, contact CDC's National Immunization Hotline at 1-800-232-2522 (English) or 1-800-232-0233 (Spanish). [Top]
Shingles (Zoster)
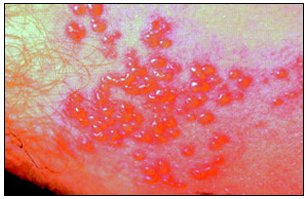
- VZV is associated with two distinct diseases: childhood chickenpox (varicella) and shingles (zoster).
- Chickenpox usually runs its course in about a week or two, but VZV is not eliminated from the body. The virus becomes dormant in sensory ganglia and may reactivate decades later to produce zoster (shingles) or herpes zoster.
- The incidence of herpes zoster in the United States is estimated to be 600,000 to one million cases per year.
- The virus may occasionally revert to its infectious state, but it is usually held in check by cell-mediated immunity (CMI).
- It is when CMI declines, such as in advanced age, lymphoma, or AIDS, that the virus reverts back to its infectious state and zoster results.
- It has been reported that blacks have a significantly lower risk of developing zoster than whites, suggesting a racial difference in susceptibility to VZV reactivation (herpes zoster).
- It has also been reported that herpes zoster is an early manifestation of HIV infection in young Africans.
- The first symptom of zoster is usually pain and is caused by nerve damage due to VZV reactivation from latently infected ganglia. It is believed that the pain is usually at the site where the lesions are about to break out.
- In rare episodes of zoster infection, pain is accompanied by flu-like symptoms (fever, malaise, chills, gastrointestinal distress, and/or headache).
- The pain, which can range from mild itching or tingling to severe pain, is followed, usually within 5 days, by swelling or redness of the skin and clusters of clear vesicles, which soon develop into blisters. The eruption of zoster is unilateral and does not cross the midline. The zoster lesions resemble varicella, but unlike varicella they evolve less rapidly and appear as grouped vesicles rather than single lesions. They may be scattered in patches or so numerous as to form a continuous band.
- Most commonly the regions affected are those supplied by the trigeminal nerve and thoracic ganglia. These areas include those around the chest and abdomen, and the eyes (ophthalmic zoster).
- Normally, new zoster lesions continue to appear for 2-3 days, and within 14 days the lesions become pustular and crusty. At this point, they no longer contain the virus.
- Though this is the normal course of zoster, complications can often occur. The most common and debilitating complication is postherpetic neuralgia (PHN).
- Another major complication of zoster is bacterial infection. This can cause severe complications because of the possibility of superficial gangrene and subsequent scarring. In the case of ophthalmic zoster, severe infection can cause corneal opacification or secondary bacterial infection.
- In general, approximately 10%-20% of the U.S. population will eventually develop one or more cases of shingles. However, herpes zoster is more common among immunocompromised persons.
- Immunocompetence declines with age, disease, and with some medical treatments, such as systemic corticosteroid therapy.
- Close to 50% of those who live beyond the age of 80 can expect to develop shingles. Of those patients with HIV infection, AIDS, lymphoma, malignancy, and other immune deficiencies and those who are recipients of bone marrow and kidney transplants, 50% can be expected to develop zoster.
- Therefore, otherwise healthy and young adults who develop zoster may wish to have themselves tested for an immunodeficiency or a malignancy (rarely, however, is zoster the only clue to such an illness). As the U.S. population ages, we may also expect an increase in the cases of herpes zoster.
Diagnosis of Shingles
- There are two ways to diagnose herpes zoster—clinical diagnosis and laboratory diagnosis.
- Clinical diagnosis involves evaluating the various symptoms of the disease. Differentiating zoster from most diseases is rather clear-cut; the difficulty arises when attempting to differentiate herpes zoster from herpes simplex .
- Laboratory tests are necessary to accurately diagnose herpes zoster.
Treatment of Shingles
- Acyclovir is a widely used drug for the treatment of herpes zoster.
- The drug reduces healing time, the appearance of new vesicles, the duration of pain and duration of viral shedding.
- Other drugs, including desciclovir, famciclovir, valaciclovir, and penciclovir--all variants of acyclovir--have also been shown to provide adequate treatment of herpes zoster.
- Oral famciclovir (500 mg or 750 mg three times daily for 7 days) is an effective and well-tolerated therapy for herpes zoster. In addition, famciclovir decreases the duration of PHN. [Top]
Postherpetic Neuralgia (PHN)
- PHN is one of the major complications of herpes zoster (shingles). This is when the patient continues to feel pain even after the skin lesions have crusted.
- The pain is often severe in the areas where the blisters occurred. The affected areas are also extremely sensitive to heat and cold. PHN is thought to result from nerve damage caused be the virus.
- Risk factors for the development of PHN may include severity of pain, significant sensory impairment and advancing age.
- Prompt treatment of acute zoster pain may decrease the incidence of PHN.
- In dealing with the chronic pain associated with PHN, analgesics can often be used. Steroid treatment is also used to reduce inflammation in the dorsal root ganglia and nerves, but its use still remains controversial. [Top]
Varicella Vaccine
- A live attenuated varicella virus (Oka strain) vaccine (VARIVAX) was
licensed in 1995 in the United States and is manufactured by Merck & Co., Inc.
This virus strain was isolated in Japan in the early 1970s from a child (Oka) with chickenpox. The virus was attenuated through sequential passages in human embryonic lung cells, embryonic guinea pig cells, and human diploid cells (WI-38). The attenuated virus was then used to immunize children against chickenpox. - Varicella vaccine is lyophilized (freeze dried). When reconstituted as directed by the package insert and stored at room temperature for 30 minutes, the virus contains more than 1,350 plaque forming units of Oka/Merck VZV in each 0.5-ml dose. Each dose also contains 12.5 mg of hydrolyzed gelatin, trace amounts of neomycin and fetal bovine serum, 25 mg of sucrose, and trace residual components of MRC-5 cells, including DNA and proteins. The vaccine does not contain preservatives.
- The seroconversion rate of varicella vaccine among susceptible children 12 months to 12 years of age is 97%. Among vaccinated persons 13 years of age or older, 78% seroconvert after the first dose of varicella vaccine, and 99% seroconvert after a second dose.
- The vaccine has proven to be effective for more than 10 years in preventing varicella. However, breakthrough infection (i.e., cases of chickenpox after vaccination) can occur (less than 1%-4.4%), usually resulting in mild illness. Healthy vaccinated persons have a minimal risk for transmitting vaccine virus to their contacts; this may be higher in vaccinees in whom a varicella-like rash develops following vaccination. Higher risk for transmission of vaccine virus has been documented among children who have both rash following vaccination and leukemia. In one study, varicella virus vaccine infection occurred in 15 (17%) of 88 exposed, healthy siblings of leukemic vaccine recipients; mild rash developed in 11 siblings.
- The incidence of herpes zoster after vaccination among otherwise healthy children is 18 per 100,000 person years of follow-up.
Distribution, Handling, and Storage of Vaccine
- The lyophilized vaccine (VARIVAX) must be stored at an average temperature of 5o F (-15o C) or lower. The vaccine should be reconstituted according to the package insert and only with the diluent supplied with the vaccine.
- The diluent should be stored separately either at room temperature or in the refrigerator.
- Once reconstituted, the vaccine should be used immediately to minimize loss of potency.
- If not used, the vaccine should be discarded (according to the guidelines for waste disposal of biohazard products) within 30 minutes after reconstitution.
Recommendations for the Use of Varicella Vaccine
- Varicella virus vaccine has been approved for use among healthy children
12 months to 12 years of age.
• Children in this age group should receive one 0.5-ml dose of vaccine subcutaneously.
• All children should be routinely vaccinated at 12-18 months of age. Varicella vaccine preferably should be administered routinely to children at the same time as MMR (Mumps, Measles, Rubella) vaccine at separate sites and with separate syringes.
• Investigational vaccine studies have suggested no notable interactions between varicella and any other vaccines that are routinely administered to young children (e.g., measles, mumps, rubella, diphtheria, tetanus, pertussis, Haemophilus influenza type b, hepatitis B virus, live and inactivated polio viruses vaccines).
• Simultaneous administration of most widely used live, attenuated and inactivated vaccines has not resulted in impaired antibody response or an increased rate of adverse events. - Varicella virus vaccine is recommended for all susceptible children by their 13th birthday.
- Varicella virus vaccine is approved for use among healthy adolescents and
adults.
• Persons 13 years of age or older should be administered two 0.5-ml doses of vaccine, subcutaneously, 4-8 weeks apart. If more than 8 weeks elapse following the first dose, the second dose can be administered without restarting the schedule.
• Vaccination is recommended for susceptible adolescents, adults, and health-care workers and family contacts of immunocompromised persons.
• Vaccination should be considered for susceptible persons: teachers of young children, day-care employees, residents and staff of institutional settings, inmates and staff of correctional settings, military personnel, and women who are not pregnant but who may become pregnant in the future.
• Vaccination should be considered for international travelers who are not immune to VZV infection.
Varicella Virus Vaccine Adverse Reactions
- Pain and redness at the injection site were the only adverse reactions that occurred significantly more often in vaccine recipients in controlled clinical trials.
- In uncontrolled clinical trials of 8,900 healthy children (12 months to 12 years of age) who received one dose of vaccine, 14.7% developed fever, 19.3% had complaints regarding the injection site, 3.4% had a mild varicella rash at the injection site, and 3.8% had a non-localized varicella rash.
- In uncontrolled clinical trials of 1,600 persons age 13 years or older who received one dose, and 955 who received two doses of varicella vaccine: 10.2% and 9.5%, respectively, developed fever; 24.4% and 32.5% had complaints regarding the injection site; 3% and 1% developed a varicella-like rash at the injection site; and 5.5% and 0.9%, respectively, developed a non-localized rash.
- During the first 12 months following vaccine licensure, more than 2.3 million doses of vaccine were distributed in the United States. The Vaccine Adverse Events Reporting System (VAERS) and the vaccine manufacturer have received a limited number of reports of serious medical events occurring within 6 weeks after vaccination, including 4 cases of encephalitis , 7 cases of ataxia, and 10 cases of erythema multiforme. Three cases of anaphylaxis have occurred within 10 minutes of vaccination. A causal relationship between the vaccine and these events has not been determined.
- Serious adverse reactions regardless of whether they are or are not suspected to have been caused by varicella vaccine, should be reported to VAERS at 800-822-7967. In addition, in some states, serious adverse reactions should be reported to the State Health Department.
Contraindications and Precautions
- Varicella virus vaccine should not be administered to persons who have a history of anaphylactic reaction to any component of the vaccine, including gelatin and neomycin.
- Vaccination of persons who have severe illness should be postponed until recovery. Vaccination is not recommended for persons who have untreated and active tuberculosis.
- Varicella virus vaccine is not licensed for use in persons who have any malignant condition.
- Varicella vaccine should not be administered to persons who have primary or acquired immunodeficiency, including immunosuppression associated with AIDS or other clinical manifestations of HIV infection, cellular immunodeficiency, hypogammaglobulinemia, and dysgammaglubulinemia.
- Varicella vaccine should not be administered to persons who have a family history of congenital or hereditary immunodeficiency in first degree relatives.
- Varicella virus vaccine should not be administered to persons receiving immunosuppressive doses of corticosteroids.
- Children who are receiving high doses of systemic steroids (2 mg/kg body weight or more of prednisone) for 2 weeks or longer may be vaccinated after the steroid therapy has been discontinued for at least 3 months.
- Vaccinees in whom vaccine-related rash develops should avoid contact with susceptible immunosuppressed persons.
- Varicella vaccine should not be administered for at least 5 months after administration of blood, plasma, immunoglobulins (IG), or VZIG.
- Vaccine recipient should avoid using salicylates (aspirin) for 6 weeks after vaccination because of the association of aspirin use and Reye’s syndrome following varicella.
- Pregnant women should not be vaccinated. Non pregnant women who are vaccinated should avoid becoming pregnant for 1 month following each injection.
Use of VZIG for Post Exposure Prophylaxis
- Varicella-zoster immune globulin (VZIG) prevents or modifies clinical illness in susceptible, immunocompromised persons who are exposed to varicella or zoster.
- VZIG is prepared from plasma obtained from healthy volunteer blood donors who are identified by routine screening to have high antibody titers to VZV. VZIG is a sterile, 10%-18% solution of the globulin fraction of human plasma, primarily immunoglobulin G (IgG) in 0.3 M glycine as a stabilizer and 1:10,000 thimerosal as a preservative. VZIG is prepared by cold ethanol precipitation, which eliminates hepatitis B virus, HIV, and other known infectious agents from the product.
- VZIG is produced by the United States Biologics Laboratories (Massachusetts) and distributed by American Red Cross regional distribution centers.
Administration of VZIG
- VZIG provides maximum benefit when administered as soon as possible after the presumed exposure, but it may be effective if administered as late as 96 hours after exposure. The protection after VZIG administration may last for a period of approximately 3 weeks.
Dosage of VZIG
- VZIG is supplied in two different dosages: the 125-U vial and the 625-U vial. The recommended dose is 125 U/10 kg (22 lbs) of body weight, up to a maximum dose of 625 U. The minimum dose is 125 U and 625 U should be sufficient to modify or prevent infection in healthy adults. Higher doses may be necessary for immunocompromised adults.
- VZIG should be administered intramuscularly, as directed by the manufacturer’s instructions. VZIG should never be used intravenously.
Indications for the Use of VZIG
- The decision to administer VZIG to a person exposed to varicella should
be based on whether:
1. The patient is susceptible to VZV infection.
2. The exposure is likely to result in infection.
3. The patient is at greater risk for complications than the general population. - Persons who receive bone-marrow transplants should be considered susceptible, regardless of prior history of varicella or varicella vaccination in themselves or in their donors.
Types of Exposure For the Use of VZIG
- Direct contact exposure of 1 hour or longer with an infectious person while indoors.
- Sharing the same hospital room with an infectious patient or prolonged direct face-to-face contact with an infectious person (e.g., health-care workers).
- Continuous exposure to household members who have varicella.
Recommendations for the Use of VZIG
- Persons less than 13 years of age:
• Immunocompromised children after substantial exposure to varicella.
• Neonates whose mothers become infected with varicella shortly before delivery.
• Postnatal exposure of neonates. - Persons 13 years of age and older:
• Immunocompromised persons who are considered susceptible and who have had substantial exposure to varicella should receive VZIG.
• pregnant women who are susceptible and who have been exposed to varicella.
• Hospital personnel who are susceptible and are exposed to varicella.
VZIG Associated Adverse Effects and Precautions
The most frequent adverse reaction following VZIG administration is local discomfort at the injection site. Pain, redness, and swelling occur at the injection site in approximately 1% of patients. Less frequent adverse events (0.2%) include gastrointestinal symptoms, malaise, headache, rash, and respiratory symptoms.
Scientific Resources Program (SRP)
National Center for Infectious Diseases (NCID)
Centers for Disease Control and Prevention, Atlanta, GA (CDC)